Answer:
P= 168258.30696 Pa
Step-by-step explanation:
Given that
Mass of water vapor m = 19.00 g
Volume of water vapor V = 2.00 L
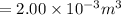
Temperature of water vapor is T = 111°C
= 384K
Molar mass of water is M = 18.0148 g/mol
Number of moles are
n = m/M
= (1.90 g)/(18.0148 g/mol)
= 0.1054 mol
Pressure inside the container is
P= nRT/V
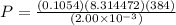
P= 168258.30696 Pa