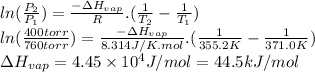Answer:
44.5 kJ/mol
Step-by-step explanation:
Propanol has a normal boiling point of T₁ = 97.8°C = 371.0 K. "Normal" refers to a pressure P₁ = 1 atm = 760 torr. At 400 torr (P₂), it has a boiling point of T₂ = 82.0°C = 355.2 K. We can find the heat of vaporization (ΔHvap) using the two point form of the Clausius-Clapeyron equation.