Answer :
(a) The heat needed when it is added at constant volume is 8.5 kJ.
(b) The heat needed when it is added at constant pressure is 14.1 kJ.
Explanation :
Heat released at constant pressure is known as enthalpy.
Heat released at constant volume is known as internal energy.
(a) The formula used for change in internal energy of the gas is:
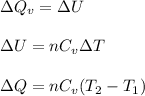
where,
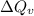 = heat at constant volume = ?
= heat at constant volume = ?
 = change in internal energy
= change in internal energy
n = number of moles of gas = 2.0 moles
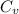 = heat capacity at constant volume for monoatomic gas =
= heat capacity at constant volume for monoatomic gas =
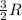
R = gas constant = 8.314 J/mole.K
 = initial temperature = 340 K
= initial temperature = 340 K
 = final temperature =
= final temperature =
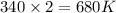
Now put all the given values in the above formula, we get:
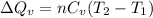
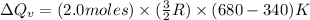
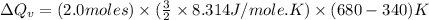
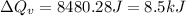
Thus, the heat needed when it is added at constant volume is 8.5 kJ.
(b) The formula used for change in enthalpy of the gas is:
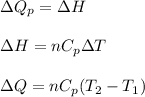
where,
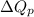 = heat at constant pressure = ?
= heat at constant pressure = ?
 = change in enthalpy energy
= change in enthalpy energy
n = number of moles of gas = 2.0 moles
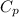 = heat capacity at constant pressure for monoatomic gas =
= heat capacity at constant pressure for monoatomic gas =
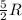
R = gas constant = 8.314 J/mole.K
 = initial temperature = 340 K
= initial temperature = 340 K
 = final temperature =
= final temperature =
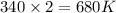
Now put all the given values in the above formula, we get:
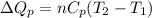
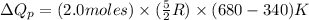
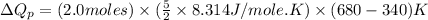
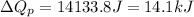
Thus, the heat needed when it is added at constant pressure is 14.1 kJ.