Answer:
The final temperature is 806.226 degrees celcius.
Step-by-step explanation:
It is given that initial conditions are as follows,
Initial volume =
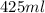
Initial temperature =
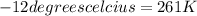
Initial pressure =
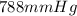
The final conditions are as follows,
Final volume =
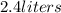
Final pressure =
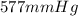
Final temperature = "T"
Let R be universal gas constant, n be number of moles..
The ideal gas equation, is as follows,
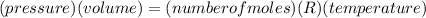
The number of moles remain constant in the process,
so equating initial number of moles = final number of moles , we get
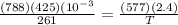
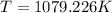 = 806.226 degrees celcius
= 806.226 degrees celcius