Answer:
The ions present in the solution is
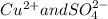 .
.
Step-by-step explanation:
The given solution is copper(II)sulfate.
The molecular formula of the solution is
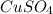 .
.
The electrolysis of copper sulfate solution has two types of ions namely cations and anions.
The dissociation of
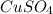 is as follows.
is as follows.
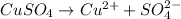
The copper ions are deposited at cathode.
The oxygen gas liberated at anode.
Ions present in the electrolyte :
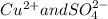 .
.
Product at cathode : Copper
Product at anode : Oxygen gas