Answer:
13.84 L of Ne gas
Step-by-step explanation:
The exercise can be resolved by applying the General Gas Law, which establishes the relationship between the Pressure, Volume and Temperature of a gas in its initial state and its final state. Mathematically, it is:
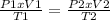
To use this equation, you need to convert the temperatures from Celsius scale to Kelvin scale (absolute scale), and to make this conversion the formula is used:
T (K) = t (° C) + 273.15
We replace the values and obtain the absolute temperatures of the initial state (T₁) and the final state (T₂)
T₁ (K) = 27.0 ° C + 273.15 = 300.15 K
T₂ (k) = 77.0 ° C + 273.15 = 350.15 K
Clearing in the equation of the general gas law V₂, and replacing the other values provided by the statement, the equation and result would be:
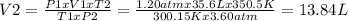
Therefore, the final volume occupied by the gas sample Ne is 13.84 L