Answer:
n = 0.594 mol
Step-by-step explanation:
Parameters of the question.
Heat = 979J
Initial Temperature (T1) = 10.6 C = 283.6 K (converting to Kelvin by adding 273)
FinalTemperature (T2) = 26.4 C = 299.4 K (converting to Kelvin by adding 273)
ΔT = T2 -T1 = 299.4 - 283.6 = 15.8K
Work done (W) = 221 J
number of moles (n) = ?
Formular to be used;
W=PΔV
From ideal gas equation,
PV = nRT
PΔV = nRΔT
where R = gas constant = 8.314 J / mol·K
Sunstituting in the work done equation,
we have W=nRΔT
Making n subject of formular,
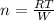
n = [8.314 * 15.8]/221
n = 131.36/221
n = 0.594 mol