Answer:
The value of free enrage that is ΔG for the overall reaction is 36.3 kJ/mol.
Step-by-step explanation:
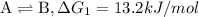 ...[1]
...[1]
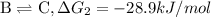 ...[2]
...[2]
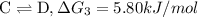 ...[3]
...[3]
To find ΔG for reaction :
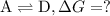 ...[4]
...[4]
By using Hess's law:
[1] - [2] - [3] = [4]
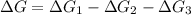
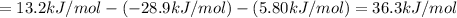
The value of free enrage that is ΔG for the overall reaction is 36.3 kJ/mol.