Answer:
non-spontaneous
Step-by-step explanation:
The reaction which shows the removal of the terminal phosphate from the ATP is shown below:
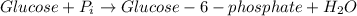
The Gibbs' free energy change of this reaction,
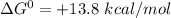
For a reaction of be spontaneous,
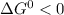
The Gibbs' free energy change is positive which means that the reaction is energetically unfavorable. or non-spontaneous.