The new pressure of the gas if the balloon is squeezed down to a volume of .6 L is 6.66 Pa
Step-by-step explanation:
Given:
 =1L
=1L
 =4 Pa
=4 Pa
 =0.6 L
=0.6 L
To find:

According to Boyles law, pressure and volume are inversely proportional. This relationship can be found only when the temperature and amount of gas remain constant.
The expression is given as,
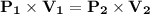
Substituting the values in the equation,
Now,
4 × 1 =
 ×0.6
×0.6
 =4/0.6
=4/0.6
= 6.66 Pa
The same expression can be used to find the change in volume when pressure is given or vice versa