Answer with Explanation:
We have to calculate the standard emf of a cell.
Consider
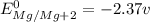
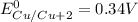
T=
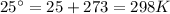
Anode half reaction:
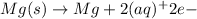
Cathode half reaction:
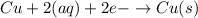
Overall reaction of cell :
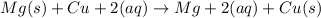
Cathode :The electrode which gains electrons.
Anode: The electrode which losses electrons.
We know that standard emf of the cell
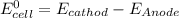
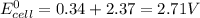
Hence, the standard emf of a cell=2.71 V