Answer:
In percentage, the sample of C-4 remains = 0.7015 %
Step-by-step explanation:
The Half life Carbon 14 = 5730 year
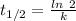
Where, k is rate constant
So,
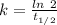
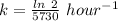
The rate constant, k = 0.000120968 year⁻¹
Time = 41000 years
Using integrated rate law for first order kinetics as:
![[A_t]=[A_0]e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/college/wgh5hifj7f12vitsa51kophgqrxxcfit2c.png)
Where,
![[A_t]](https://img.qammunity.org/2020/formulas/chemistry/college/wbj92t0z4axifcyqa24z3ary269op2iva8.png) is the concentration at time t
is the concentration at time t
![[A_0]](https://img.qammunity.org/2020/formulas/chemistry/college/izynxfnwyud2ghdog9l8ny0mhzwshbud6r.png) is the initial concentration
is the initial concentration
So,
![\frac {[A_t]}{[A_0]}=e^(-0.000120968* 41000)](https://img.qammunity.org/2020/formulas/chemistry/college/8be7f5yny9dumuuzglatwoa5wto7zt0bwu.png)
![\frac {[A_t]}{[A_0]}=0.007015](https://img.qammunity.org/2020/formulas/chemistry/college/kc6azbztw8per2xp1wdvmqcd0r3tg0bypj.png)
In percentage, the sample of C-4 remains = 0.7015 %