Answer:
8.625 grams of a 150 g sample of Thorium-234 would be left after 120.5 days
Step-by-step explanation:
The nuclear half life represents the time taken for the initial amount of sample to reduce into half of its mass.
We have given that the half life of thorium-234 is 24.1 days. Then it takes 24.1 days for a Thorium-234 sample to reduced to half of its initial amount.
Initial amount of Thorium-234 available as per the question is 150 grams
So now we start with 150 grams of Thorium-234
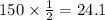
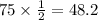
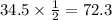
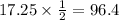
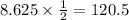
So after 120.5 days the amount of sample that remains is 8.625g
In simpler way , we can use the below formula to find the sample left
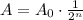
Where
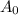 is the initial sample amount
is the initial sample amount
n = the number of half-lives that pass in a given period of time.