Answer: The mass of deposited cadmium is 23.9g
Step-by-step explanation:
According to Faraday Law of Electrolysis, the mass of substance deposited at the electrode is directly proportional to the quantity of electricity passed through the electrolyte.
Faraday has found that the amount of electricity needed to liberate one gm eq. of substance from an electrolyte is 96500C.
Given that
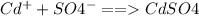
time (t) =1.55min *60 =93seconds
Current (I) =221A
Q= It = 221 * 93 =20553C
Molar mass of CdS04 =112.41
96500C will liberate 112.41g
20553C will liberate Xg
Xg= (20553*112.41)/96500
=23.9g
Therefore , the amount of Cd deposited at the electrode is 23.9g