Answer:
Part -A
The change in the entropy from largest to smallest is 3>2=4 >1 >5>6.
Part-B
Change in entropy over a complete cycle is 1 =2 = 3= 4 = 5 = 6
Step-by-step explanation:
Part -A
The change in entropy for the reversible process that transfers the heat energy 'Q' at temperature 'T' is
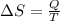
1)
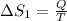
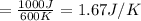
2)
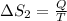
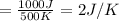
3)
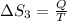
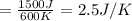
4)
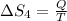
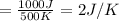
5)
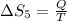
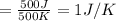
6)
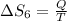
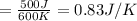
Therefore, The change in the entropy from largest to smallest is 3>2=4 >1 >5>6.
Part-B
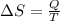
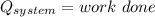
Initial and final states of complete cycle are equal. after complete the one cycle the system returns to the original state. So, the change in the entropy will be the zero
Therefore, Change in the overall cycle is 1 =2 = 3= 4 =5=6