Answer:
2475042
Step-by-step explanation:
P = Pressure = [/tex]1\times 10^{-8}\ N/m^2[/tex]
V = Volume = 1 cm³
T = Temperature = 19.5 °C
R = Gas constant = 8.341 J/mol K
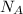 = Avogadro's number =
= Avogadro's number =

n = Amount of substance
From ideal gas law we have
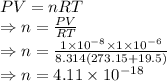
Number of atoms is given by
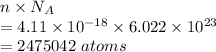
The number of atoms in this vacuum is 2475042