Answer:
12 g.
Step-by-step explanation:
We have to make 100 ml of 2.0M acetic acid.
no. of moles of acetic acid required for this will be calculated by the formula,
Molarity =
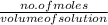
Here,
volume of solution = 0.1 L
Molarity = 2.0M
So, no. of moles = 0.2
Molecular weight of acetic acid = 60 g.
So,
Weight of acetic acid required = 0.2×60 g = 12 g.