Answer : The option (c) is not a conjugate acid-base pair.
Explanation :
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
Or we can say that, conjugate acid is proton donor and conjugate base is proton acceptor.
(a) The equilibrium reaction will be,
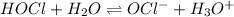
In this reaction,
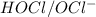 are act as a conjugate acid-base pair.
are act as a conjugate acid-base pair.
(b) The equilibrium reaction will be,
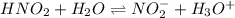
In this reaction,
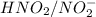 are act as a conjugate acid-base pair.
are act as a conjugate acid-base pair.
(c) The equilibrium reaction will be,
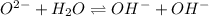
In this reaction,
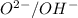 are not act as a conjugate acid-base pair.
are not act as a conjugate acid-base pair.
(d) The equilibrium reaction will be,
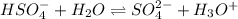
In this reaction,
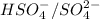 are act as a conjugate acid-base pair.
are act as a conjugate acid-base pair.
(e) The equilibrium reaction will be,
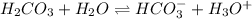
In this reaction,
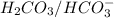 are act as a conjugate acid-base pair.
are act as a conjugate acid-base pair.
Hence, from this we conclude that, the option (c) is not a conjugate acid-base pair but it is a act as conjugate base-acid pair.