Answer:
The heat of the given reaction is -23.0 kJ.
Step-by-step explanation:
We are given with ;
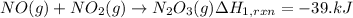 ..[1]
..[1]
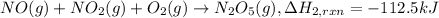 ..[2]
..[2]
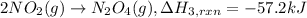 ..[3]
..[3]
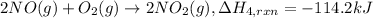 ..[4]
..[4]
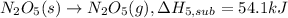 ..[5]
..[5]
To find heat of reaction:
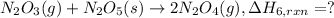 ..[6]
..[6]
Using Hess's law:
[5] +2 × [3] + [4] - [2] - [1] = [6]
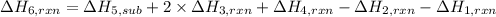
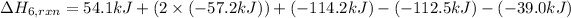
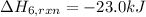
The heat of the given reaction is -23.0 kJ.