Answer:
182 grams of oxygen gas must react to form 3.80 mol of aluminum oxide.
Step-by-step explanation:
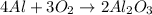
Moles of aluminum oxide = 3.80 mole
According to reaction, 2 moles of aluminum oxide is obtained from 3 moles of oxygen gas.
Then 3.80 moles of aluminum oxide will be obtained from:
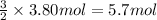 of oxygen gas.
of oxygen gas.
Mass of 3.80 moles of oxygen = 5.7 mol × 32 g/mol = 182.4 g ≈ 182 g
182 grams of oxygen gas must react to form 3.80 mol of aluminum oxide.