The theoretical yield of sodium chloride formed from the reaction of 3.3 g of hydrochloric acid and 6.7 g of sodium hydroxide is 5.3 g
How to obtain the theoretical yield of sodium chloride?
We shall begin by determining the limiting reactant for the reaction of 3.3 g of hydrochloric acid and 6.7 g of sodium hydroxide. This is shown below:
HCl + NaOH -> NaCl + H₂O
- Molar mass of HCl = 36.5 g/mol
- Mass of HCl from the balanced equation = 1 × 36.5 = 36.5 g
- Molar mass of NaOH = 40 g/mol
- Mass of NaOH from the balanced equation = 1 × 40 = 40 g
From the balanced equation above,
36.5 g of HCl reacted with 40 g of NaOH
Therefore,
3.3 g of HCl will react with = (3.3 × 40) / 36.5 = 3.6 g of NaOH
Now, we can see that only 3.6 g of NaOH reacted with 3.3 g of HCl
Thus, the limiting reactant is HCl
Finally, we shall calculate the theoretical yield of sodium chloride. Details below:
HCl + NaOH -> NaCl + H₂O
- Molar mass of HCl = 36.5 g/mol
- Mass of HCl from the balanced equation = 1 × 36.5 = 36.5 g
- Molar mass of NaCl = 58.5 g/mol
- Mass of NaOH from the balanced equation = 1 × 58.5 = 58.5 g
- Theoretical yield of sodium chloride =?
From the balanced equation above,
36.5 g of HCl reacted to produce 58.5 of NaCl
Therefore,
3.3 g of HCl will react to produce =
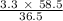 = 5.3 g of NaCl
= 5.3 g of NaCl
In conclusion, theoretical yield of sodium chloride is 5.3 g
Complete question:
Aqueous hydrochloric acid reacts with solid sodium hydroxide to produce aqueous sodium chloride and liquid water.
What is the theoretical yield of sodium chloride formed from the reaction of 3.3 g of hydrochloric acid and 6.7 g of sodium hydroxide.? Round your answer to significant figure