Answer: The partial pressure of ammonia is 0.464 atm
Step-by-step explanation:
For the given chemical equation:
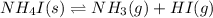
Initial: 1
At eqllm: 1-x x x
The expression for
 for the following equation is:
for the following equation is:
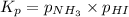
The partial pressures of solids and liquids are taken as 1 in the equilibrium expression.
We are given:
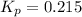
Putting values in above equation, we get:
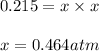
Hence, the partial pressure of ammonia is 0.464 atm