Answer: d) ΔS is positive and ΔH is negative
Step-by-step explanation:
According to Gibb's equation:
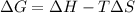
 = Gibbs free energy
= Gibbs free energy
 = enthalpy change
= enthalpy change
 = entropy change
= entropy change
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
a) ΔS is negative and ΔH is positive
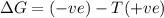
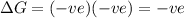
Reaction is spontaneous at all temperatures.
b) ΔS is positive and ΔH is positive
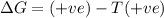
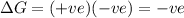
Reaction is spontaneous at high temperatures.
c) ΔS is negative and ΔH is negative
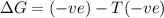
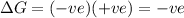
Reaction is spontaneous at low temperatures.
d) ΔS is positive and ΔH is negative
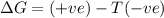
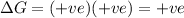
Reaction is non spontaneous or thermodynamically unfavored at all temperatures.