Answer:
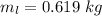
Step-by-step explanation:
Given:
- initial temperature of water(lemonade),
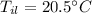
- mass of ice,
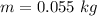
- initial temperature of ice,
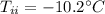
- final temperature of the mixture,
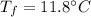
- specific heat capacity of ice,
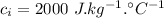
- specific heat capacity of water,
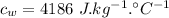
- Latent heat of fusion of ice,
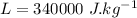
For the whole ice to melt in lemonade and result a temperature of 11.8°C the total heat lost by the lemonade will be equal to the total heat absorbed by the ice to come to 0°C from -10.2°C along with the latent heat absorbed in the melting of ice at 0°C and the heat absorbed by the ice water of 0°C to reach a temperature of 11.8°C.
Now, mathematically:
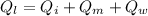
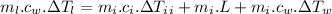
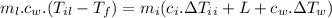
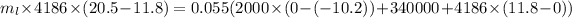
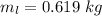 (mass of lemonade)
(mass of lemonade)