Answer: D) 1.00 g
Step-by-step explanation:
According to the Avogadro's law, the volume of gas is directly proportional to the number of moles of gas at same pressure and temperature. That means,
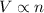
or,
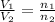
where,
 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L
 = final volume of gas = 3.00 L
= final volume of gas = 3.00 L
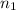 = initial moles of gas =
= initial moles of gas =
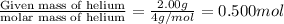
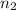 = final moles of gas = ?
= final moles of gas = ?
Now we put all the given values in this formula, we get
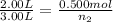
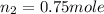
Mass of helium =
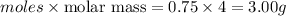
Thus mass of helium added = (3.00-2.00) g = 1.00 g