Answer:
The variation of pressure is 0.
Step-by-step explanation:
Methane combustion reaction:
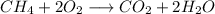
The pressure change in gaseous state reactions at constant T y V is proportional to the change in the number of mol of gas in total.
As can be seen in the reaction above we have 3 moles of gas in the reactants and 3 in the products, so the variation of moles is 0. Therefore, the variation of pressure is also 0.