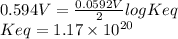Answer:
Keq = 1.17 × 10²⁰
Step-by-step explanation:
Let's consider the following redox reaction.
Cu²⁺(aq) + Ni(s) → Cu(s) + Ni²⁺(aq)
We can identify 2 half-reactions.
Cathode (reduction): Cu²⁺(aq) + 2 e⁻ → Cu(s) E°red = 0.337 V
Anode (oxidation): Ni(s) → Ni²⁺(aq) + 2 e⁻ E°red = -0.257 V
The standard cell potential (E°) is the difference between the standard reduction potential of the cathode and the standard reduction potential of the anode.
E° = E°red, cat - E°red, an = 0.337 V - (-0.257V) = 0.594 V
We can calculate the equilibrium constant (Keq) using the following expression.
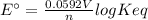
where,
n are the moles of electrons transferred