Answer:
there are two Mo atoms per seven Fe atoms
Step-by-step explanation:
The metal X = Molybdenum
Metal Y = Iron
The atomic weight of
Mo = 96 g/ mol
Fe = 56 g/mol
The weight ratio of given metals is
Mo = 33%
Fe =67%
Let us calculate the moles of each metal in 100g of sample.
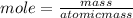
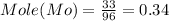
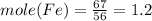
Thus metal atom ratio =
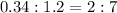
Thus there are two Mo atoms per seven Fe atoms