Answer: Option (A) is the correct answer.
Step-by-step explanation:
Viscosity is defined as the ability of a liquid to resist its flow. When a substance has high viscosity then it is known as a viscous substance.
Also, more is the mass of a substance more will be its resistance to move from one place to another.
- Mass of hexane is 86.18 g/mol
- Mass of
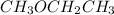 is 60.09 g/mol
is 60.09 g/mol
- Mass of
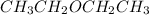 is 74.12 g/mol
is 74.12 g/mol
- Mass of
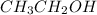 is 40.06 g/mol
is 40.06 g/mol
Mass of
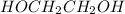 is 62.07 g/mol
is 62.07 g/mol
Therefore, out of the given options viscosity of hexane is the greatest.