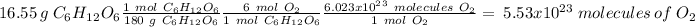Answer:
a) 40 %
b)
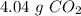
c)
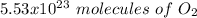
Step-by-step explanation:
For a) we will have to calculate the molar mass of
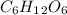 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
C => 12*(6) = 72
H => 1*(12) = 12
O => 6*(16) = 96
Molar mass = 180 g/mol
Then we can calculate the percentage by mass:
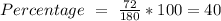
For b) we have to start with the reaction of glucose:
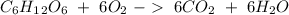
Then we have to convert the grams of glucose to moles, the moles of glucose to moles of carbon dioxide and finally the moles of carbon dioxide to grams. To do this we have to take into account the following conversion ratios:
-) 180 g of glucose = 1 mol glucose
-) 1 mol glucose = 6 mol carbon dioxide
-) 1 mol carbon dioxide = 44 g carbon dioxide
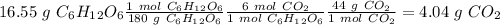
For C, we have to start with the conversion from grams of glucose to moles, the moles of glucose to moles of oxygen and finally the moles of oxygen to molecules. To do this we have to take into account the following conversion ratios:
-) 180 g of glucose = 1 mol glucose
-) 1 mol glucose = 6 mol oxygen
-) 1 mol oxygen = 6.023x10^23 molecules of O2