To solve this problem it is necessary to apply the concepts related to the conservation of energy and heat transferred in a body.
By definition we know that the heat lost must be equal to the heat gained, ie
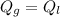
Where,
Q = Heat exchange
The heat exchange is defined as
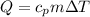
Where,
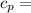 Specific heat
Specific heat
m = mass
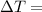 Change in Temperature
Change in Temperature
Therefore replacing we have that
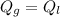
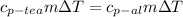
Replacing with our values we have that
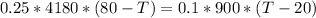
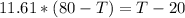
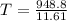
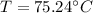
Therefore the highest possible temperature of the spoon when you finally take it out of the cup is 75.24°C