Answer:
21.21°C will be the final temperature of the solution in a coffee cup calorimeter.
Step-by-step explanation:

 = enthalpy change = -57.2 kJ/mol of NaOH
= enthalpy change = -57.2 kJ/mol of NaOH
Moles of sodium hydroxide = n
Molarity of the NaOH = 0.250 M
Volume of NaOH solution = V = 50.00 mL = 0.050 L
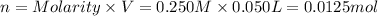
Moles of HCl = n'
Molarity of the HCl= 0.250 M
Volume of HCl solution = V' = 50.00 mL = 0.050 L
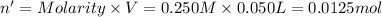
Since 1 mole of Hcl reacts with 1 mole of NaoH. Then 0.0125 mole of HCl will react with 0.0125 mole of NaOH.
The enthalpy change during the reaction.
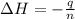
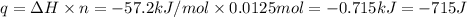
q = heat released on reaction= -715 J
now, we calculate the heat gained by the solution.:
Q= -q = -(-715 J) = 715 J
m = mass of the solution = ?
Volume of the solution formed by mixing, v = 50.00 ml + 50.00 mL = 100.00 mL
Density of the solution = density of water = d = 1 g/mL
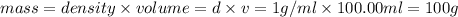
m = 100 g
q = heat gained = ?
c = specific heat =
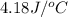
 = final temperature = ?
= final temperature = ?
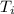 = initial temperature =
= initial temperature =
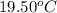
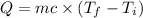
Now put all the given values in the above formula, we get:
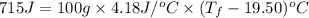
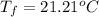
21.21°C will be the final temperature of the solution in a coffee cup calorimeter.