None of the compounds are insoluble in water out of given options.
Option: A
Step-by-step explanation:
Solubility of any compound is favored in water when it is bonded ionically or show ionic behavior Polarity of water , which is unequal sharing of electrons among its atom actually allows it to be a most popular solvent.
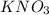 is an inorganic salt and carry ions as
is an inorganic salt and carry ions as
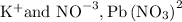 is an inorganic compound showcase ionic behavior as
is an inorganic compound showcase ionic behavior as
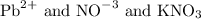 is also an inorganic and ionic salt break as
is also an inorganic and ionic salt break as
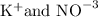 . Here when polar or ionic compound is added into water, they break up in smaller components and dissolve by connecting positive ion of solute with negative ion of solvent and negative ion of solute with positive ion of solvent.
. Here when polar or ionic compound is added into water, they break up in smaller components and dissolve by connecting positive ion of solute with negative ion of solvent and negative ion of solute with positive ion of solvent.