Answer:
a) ΔV = 2,118 10⁻⁸ m³ b) ΔR= 0.0143 cm
Step-by-step explanation:
a) For this part we use the concept of density
ρ = m / V
As we are told that 1 carat is 0.2g we can make a rule of proportions (three) to find the weight of 2.8 carats
m = 2.8 Qt (0.2 g / 1 Qt) = 0.56 g = 0.56 10-3 kg
V = m / ρ
V = 0.56 / 3.52
V = 0.159 cm3
We use the relation of the bulk module
B = P / (Δv/V)
ΔV = V P / B
ΔV = 0.159 10⁻⁶ 58 10⁹ /4.43 10¹¹
ΔV = 2,118 10⁻⁸ m³
b) indicates that we approximate the diamond to a sphere
V = 4/3 π R³
For this part let's look for the initial radius
R₀ = ∛ ¾ V /π
R₀ = ∛ (¾ 0.159 /π)
R₀ = 0.3361 cm
Now we look for the final volume and with this the final radius
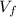 = V + ΔV
= V + ΔV
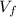 = 0.159 + 2.118 10⁻²
= 0.159 + 2.118 10⁻²
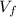 = 0.18018 cm3
= 0.18018 cm3
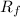 = ∛ (¾ 0.18018 /π)
= ∛ (¾ 0.18018 /π)
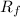 = 0.3504 cm
= 0.3504 cm
The radius increment is
ΔR =
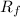 - R₀
- R₀
ΔR = 0.3504 - 0.3361
ΔR= 0.0143 cm