Step-by-step explanation:
Non-metals are the species which tend to gain electrons from a donor atom in order to attain stability.
Therefore, when a non-metal reacts with another non-metal then there will always be formation of a covalent bond. As covalent bonds are formed due to sharing of electrons.
For example,
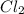 is formed by sharing of electrons.
is formed by sharing of electrons.
On the other hand, when a non-metal reacts with a metal then there will be formation of an ionic bond.
For example, LiCl is formed by transfer of electron from Li to Cl atom.
Thus, we can conclude that atoms of most non-metals share electrons and become bonded together into a molecule.