Answer:
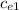 = 128.3 J / kg ° C
= 128.3 J / kg ° C
Step-by-step explanation:
In this exercise we will use that the expression for heat is
Q = m
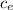 ΔT
ΔT
As they indicate that there are no losses with the medium, the heat transferred by the tungsten is equal to the heat absorbed by the water plus the calorimeter
Q assigned = QAbsorbed
Q hot = Q cold + Q calorimeter
The mass of tungsten (m₁ = 69.12 10⁻³ kg) with an initial temperature (T₁ = 98.93°C),
The mass of water (m₂ = 85.45 10⁻³ kg) at a temperature (T₂ = 23.82°C),
a calorimeter constant (C = 1.56 J/ °C)
m₁
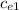 (T₁ -
(T₁ -
 ) = (m₂
) = (m₂
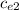 + C) (
+ C) (
 - T₂)
- T₂)
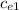 = (m₂ ce2 + C) (
= (m₂ ce2 + C) (
 -T₀) / (m₁ (T₁-
-T₀) / (m₁ (T₁-
 )
)
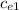 = (85.45 10-3 4186 + 1.56) (25.63 - 23.82) / (69.12 10-3 (98.93 - 25.63))
= (85.45 10-3 4186 + 1.56) (25.63 - 23.82) / (69.12 10-3 (98.93 - 25.63))
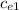 = (357.69 + 1.56) 1.81 / (69.12 10-3 73.3)
= (357.69 + 1.56) 1.81 / (69.12 10-3 73.3)
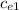 = 650.24 / 5.0665
= 650.24 / 5.0665
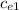 = 128.3 J / kg ° C
= 128.3 J / kg ° C