Answer:NaOH is the limiting reagent.
Step-by-step explanation:
The limiting reagent is the reactant that is totally consumed when the reaction is complete.
The given reaction is
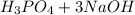 →
→
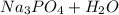
The reaction says that every mole of
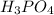 requires
requires
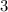 moles of
moles of
 .
.
Given that number of moles of
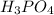 is
is
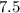
So,
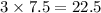 moles of
moles of
 is required.
is required.
But only
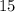 moles of
moles of
 is available.
is available.
This means that
 will be completely consumed.
will be completely consumed.
So,
 is the limiting reagent.
is the limiting reagent.