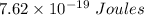Answer:
Energy of light is
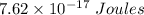
Step-by-step explanation:
Given:
Wavelength
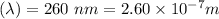
Also we know the speed of light
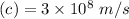
To calculate frequency of light
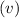 , divide speed of light
, divide speed of light
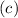 by Wavelength
by Wavelength
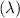
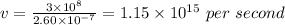
Hence frequency is
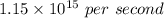
Now, To calculate energy (E), we need to multiply planks's constant
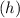 with the frequency of light
with the frequency of light
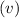 .
.
Also Plank's Constant
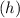 =
=
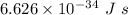
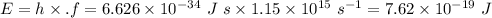
Hence Energy of light is