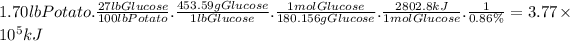Answer:
305 g of CO₂
3.77 × 10⁵ kJ
Step-by-step explanation:
Let's consider the global reaction for photosynthesis.
6 CO₂(g) + 6 H₂O(l) → C₆H₁₂O₆(g) + 6 O₂(g) ΔHrxn = 2802.8 kJ
A 1.70 lb sweet potato is approximately 73% water by mass. If the remaining mass is made up of carbohydrates derived from glucose (MW = 180.156 g/mol), how much carbon dioxide (MW = 44.01 g/mol) was needed to grow this sweet potato?
Let's consider the following relations:
- The potato is 100%-73%=27% glucose by mass.
- 1 lb = 453.59 g.
- 6 moles of CO₂ produce 1 mole of glucose.
- The molar mass of glucose is 180.156 g/mol.
- The molar mass of carbon dioxide is 44.01 g/mol.
Then, for a 1.70 lb potato:
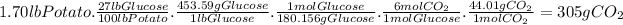
How much light energy does it take to grow the 1.70 lb. sweet potato if the efficiency of photosynthesis is 0.86%?
According to the enthalpy of the reaction, 2802.8 kJ are required to produce 1 mole of glucose. Then, for a 1.70 lb potato: