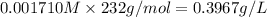Step-by-step explanation:
1)
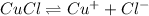
S S
Solubility product of the copper(I) chloride =
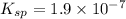
The expression of solubility product is given as :
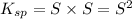
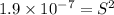
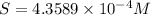
Concentration of copper(I) ions =
![[Cu^+]=4.3589* 10^(-4) M](https://img.qammunity.org/2020/formulas/chemistry/college/zecnoa8c9zhdkhdznqo2wa4a9y5wuyvixg.png)
Concentration of chloride ions =
![[Cl^-]=4.3589* 10^(-4) M](https://img.qammunity.org/2020/formulas/chemistry/college/65nyer54pwci2k2lv693g5t08w0wh6k2vv.png)
Molar solubility of CuCl =
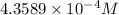
Solubility of CuCl =
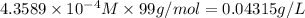
2)
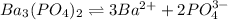
3S 2S
Solubility product of the Barium phosphate =
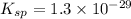
The expression of solubility product is given as :
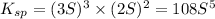
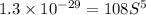
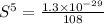
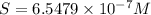
Concentration of barium ions =
![[Ba^(2+)]=3* 6.5479* 10^(-7) M =1.9643* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/college/kq3tbg3spy2bayd7mhz5loazkbriy2wel5.png)
Concentration of phosphate ions =
![[PO_4^(3-)]=2* 6.5479* 10^(-7) M =1.3096* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/college/j3mq1fdbet7729r8z0ky2ya0nmdaf8ebuw.png)
Molar solubility of
])/(3)](https://img.qammunity.org/2020/formulas/chemistry/college/jik4i1tidvzsx4o9s3soiynyu2g194swuu.png)

Solubility of
 :
:
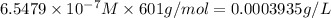
3)
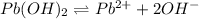
S 2S
Solubility product of the lead(II) oxide =
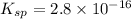
The expression of solubility product is given as :
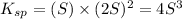
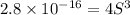
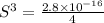
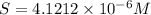
Concentration of lead(II) ions =
![[Pb^(2+)]=1* 4.1212* 10^(-6) M M =4.1212* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/college/ke82v7mqkvdmau0c6m228pbcl6jai2iwmw.png)
Concentration of hydroxide ions =
![[PO_4^(3-)]=2* 4.1212* 10^(-6) M=8.2425* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/college/iw27tvpq7s02bo155v9r92ek97xffz456f.png)
Molar solubility of
![[Pb(OH)_2]=[Pb^(2+)]=4.1212* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/college/rq3k4xtgqus9ophr7bykbjx2vxcmqg64fs.png)
Solubility of
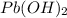 :
:

4)
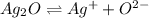
2S S
Solubility product of the lead(II) oxide =

The expression of solubility product is given as :

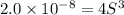
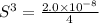

Concentration of silver ions =
![[Ag^(+)]=2* 0.001710 M =0.003420 M](https://img.qammunity.org/2020/formulas/chemistry/college/opkm0bsn7rej5rf7lp2dnogndhvjrj7njj.png)
Concentration of hydroxide ions =
![[OH^(-)]=1* 0.001710 M=0.001710 M](https://img.qammunity.org/2020/formulas/chemistry/college/663fynwlc9xmalm6b4ufqgxklj8ulu9h3d.png)
Molar solubility of
![[Ag_2O]=([Ag^(+)])/(2)](https://img.qammunity.org/2020/formulas/chemistry/college/9lfnm6suhshtlye409v8ztixs7eejwt9an.png)
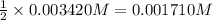
Solubility of
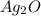 :
: