To solve the problem it is necessary to apply the concepts related to the conservation of energy through the heat transferred and the work done, as well as through the calculation of entropy due to heat and temperatra.
By definition we know that the change in entropy is given by
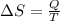
Where,
Q = Heat transfer
T = Temperature
On the other hand we know that by conserving energy the work done in a system is equal to the change in heat transferred, that is
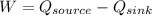
According to the data given we have to,
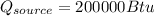
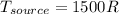
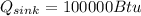
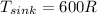
PART A) The total change in entropy, would be given by the changes that exist in the source and sink, that is
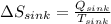
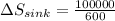
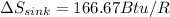
On the other hand,
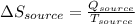
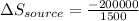
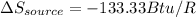
The total change of entropy would be,
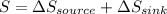
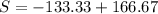
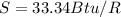
Since
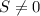 the heat engine is not reversible.
the heat engine is not reversible.
PART B)
Work done by heat engine is given by
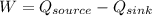
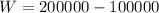
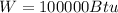
Therefore the work in the system is 100000Btu