Step-by-step explanation:
Using Reydberg's equation, we will calculate the energy emitted by the photon for the given transitions.
The equation is as follows.
![\Delta E = -2.178 * 10^(-18) J * (Z)^(2)[(1)/(n^(2)_(2)) - (1)/(n^(2)_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/8pi99cfnmzt8zlyey7nh8q83sd3v353zf7.png)
1). For
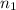 = 7,
= 7,
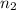 = 1, Z for H = 1
= 1, Z for H = 1
![\Delta E = -2.178 * 10^(-18) J * (Z)^(2)[(1)/(n^(2)_(2)) - (1)/(n^(2)_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/8pi99cfnmzt8zlyey7nh8q83sd3v353zf7.png)
=
![-2.178 * 10^(-18) J * (1)^(2)[(1)/((1)^(2)) - (1)/((7)^(2))]](https://img.qammunity.org/2020/formulas/chemistry/college/gh88afo4z4rjrvwq3iodxrwghe6semuux0.png)
=
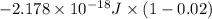
=

As, E =
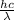
Putting the given values and calculate the wavelength as follows.
E =
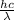
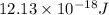 =
=
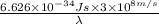
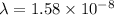 m
m
=
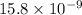 m
m
= 15.8 nm (as 1 m =
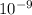 nm)
nm)
2). For
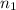 = 7,
= 7,
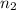 = 5, Z for H = 1
= 5, Z for H = 1
![\Delta E = -2.178 * 10^(-18) J * (Z)^(2)[(1)/(n^(2)_(2)) - (1)/(n^(2)_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/8pi99cfnmzt8zlyey7nh8q83sd3v353zf7.png)
=
![-2.178 * 10^(-18) J * (1)^(2)[(1)/((5)^(2)) - (1)/((7)^(2))]](https://img.qammunity.org/2020/formulas/chemistry/college/1vzl0jueuvml3hdpww0gx9xe6qeouh3elu.png)
=
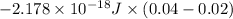
=
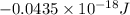
As, E =
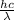
Putting the given values and calculate the wavelength as follows.
E =
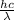
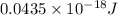 =
=
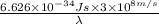
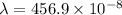 m
m
= 45.6 nm
3). For
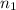 = 1,
= 1,
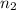 = 7, Z for H = 1
= 7, Z for H = 1
![\Delta E = -2.178 * 10^(-18) J * (Z)^(2)[(1)/(n^(2)_(2)) - (1)/(n^(2)_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/8pi99cfnmzt8zlyey7nh8q83sd3v353zf7.png)
=
![-2.178 * 10^(-18) J * (1)^(2)[(1)/((7)^(2)) - (1)/((1)^(2))]](https://img.qammunity.org/2020/formulas/chemistry/college/z6evsbhcfiz3dmfb4r7ydvssop6mzrs0qa.png)
=
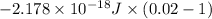
=
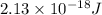
As, E =
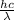
Putting the given values and calculate the wavelength as follows.
E =
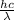
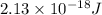 =
=
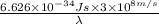
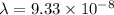 m
m
= 93.3 nm
4). For
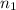 = 7,
= 7,
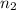 = 6, Z for H = 1
= 6, Z for H = 1
![\Delta E = -2.178 * 10^(-18) J * (Z)^(2)[(1)/(n^(2)_(2)) - (1)/(n^(2)_(1))]](https://img.qammunity.org/2020/formulas/chemistry/college/8pi99cfnmzt8zlyey7nh8q83sd3v353zf7.png)
=
![-2.178 * 10^(-18) J * (1)^(2)[(1)/((6)^(2)) - (1)/((7)^(2))]](https://img.qammunity.org/2020/formulas/chemistry/college/v3r2zh3c4n7xwq9y1k9ajx0yo7lpm41wfn.png)
=
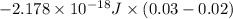
=
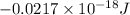
As, E =
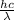
Putting the given values and calculate the wavelength as follows.
E =
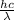
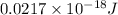 =
=
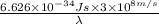
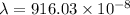 m
m
= 9160.3 nm
Thus, we can conclude that transition from n = 7 to n = 6 will emit a photon with the longest wavelength.