Answer:
446.7 °C
Step-by-step explanation:
We are given with two volumes and one temperature, we have to find another temperature.
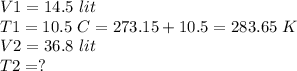
Using Charles-volume temperature law:
This law states that the volume of a given amount of gas held at a constant pressure is directly proportional to kelvin temperature.
Or,
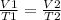
Plugging the given values.
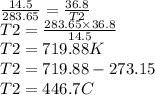
Thus the final temperature is 446.7 °C