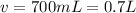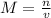Answer:1.04M
Step-by-step explanation:
Molarity is defined as the ratio of number of moles of solute per litre of solution.
Let molarity be
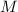 .
.
Given mass of
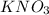
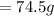
Weight of
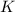 is
is
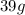
Weight of
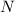 is
is
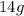
Weight of
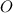 is
is
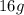
Molar mass of
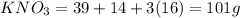
Let
 be the number of moles of solute.
be the number of moles of solute.
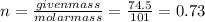
Let
 be the volume of solution.
be the volume of solution.
Given,