Answer:
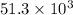 kJ
kJ
Step-by-step explanation:
The thermochemical equation for decomposition of ammonium nitrate is:
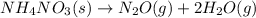


Given mass= 50.0 kg =
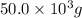 (1kg=1000g)
(1kg=1000g)
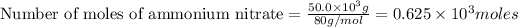
According to stoichiometry:
1 mole of
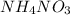 gives = 82.1 kJ of heat
gives = 82.1 kJ of heat
Thus
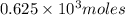 of
of
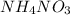 give =
give =
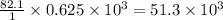 kJ of heat
kJ of heat
Thus
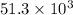 kJ of heat is evolved from the decomposition of 50.0 kg of ammonium nitrate.
kJ of heat is evolved from the decomposition of 50.0 kg of ammonium nitrate.