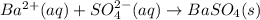Step-by-step explanation:
a.
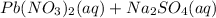 → ?
→ ?
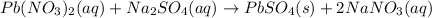
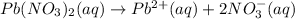
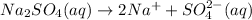
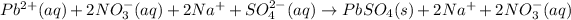
Removing common ions from both sides, we get the net ionic equation:
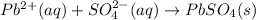
b.
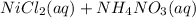 →
→
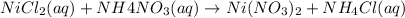
No precipitation is occuring.
c.
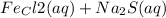 →
→
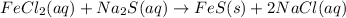
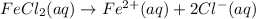
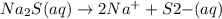
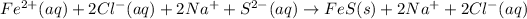
Removing common ions from both sides, we get the net ionic equation:
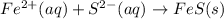
d.
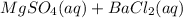 →
→
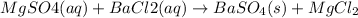
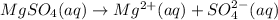
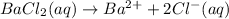
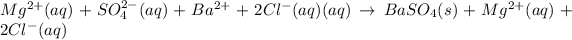
Removing common ions from both sides, we get the net ionic equation: