Answer: a)
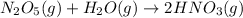
b) 3.8608 moles
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The balanced reaction for dinitrogen pentoxide gas with water to produce nitric acid is:
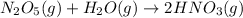

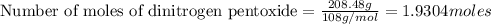
According to stoichiometry:-
As nitric acid is the limiting reagent as it limits the formation of product.
1 mole of
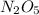 gives = 2 moles of
gives = 2 moles of
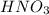
Thus 1.9304 moles of
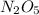 give =
give =
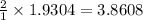 moles of
moles of
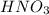
Thus 3.8608 moles of
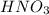 can be produced.
can be produced.