Answer:
The correct option is (e).
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 ..[1]
..[1]
For chlorine :
Given mass of chlorine = 5 g
Molar mass of chlorine = 35.5 g/mol
Putting values in equation 1, we get:
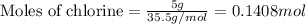
For copper :
Given mass of copper = 5 g
Molar mass of copper= 63.5 g/mol
Putting values in equation 1, we get:
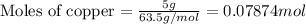
For calcium :
Given mass of calcium= 5 g
Molar mass of calcium= 40 g/mol
Putting values in equation 1, we get:
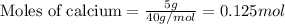
For lithium :
Given mass of lithium= 5 g
Molar mass of lithium = 7 g/mol
Putting values in equation 1, we get:
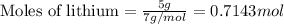
5 grams of lithium has highest number of moles.Hence, option (e) is correct.