Answer:
Resonance structures arise due to back bonding between vacant 3p orbital on Al atom and three lone pairs are present on each I atom.
Step-by-step explanation:
In Lewis structure of
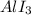 (with incomplete octet), there is a vacant 3p orbital on Al atom and three lone pairs are present on each I atom.
(with incomplete octet), there is a vacant 3p orbital on Al atom and three lone pairs are present on each I atom.
So, a back bonding is possible between these lone pair and vacant orbital. Three back bonding from three different I atoms create three resonance structures of
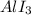 .
.
In these resonance structures, octet of Al gets satisfied as Al shares a new covalent bond.
Resonance structures are shown below.