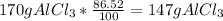Answer:
147 g of
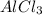 are produced with 86.52% yield.
are produced with 86.52% yield.
Step-by-step explanation:
1. Write the balanced chemical reaction for the aluminum consumed in the presence of copper II chloride dihydrate:
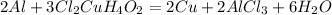
2. Calculate the maximum quantity of
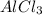 that can be produced:
that can be produced:
The limiting reagent is the Al, because the problem says that there are excess of copper II chloride dihydrate
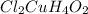
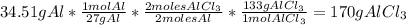
3. Calculate the quantity of
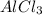 produced ith 86.52% yield:
produced ith 86.52% yield: